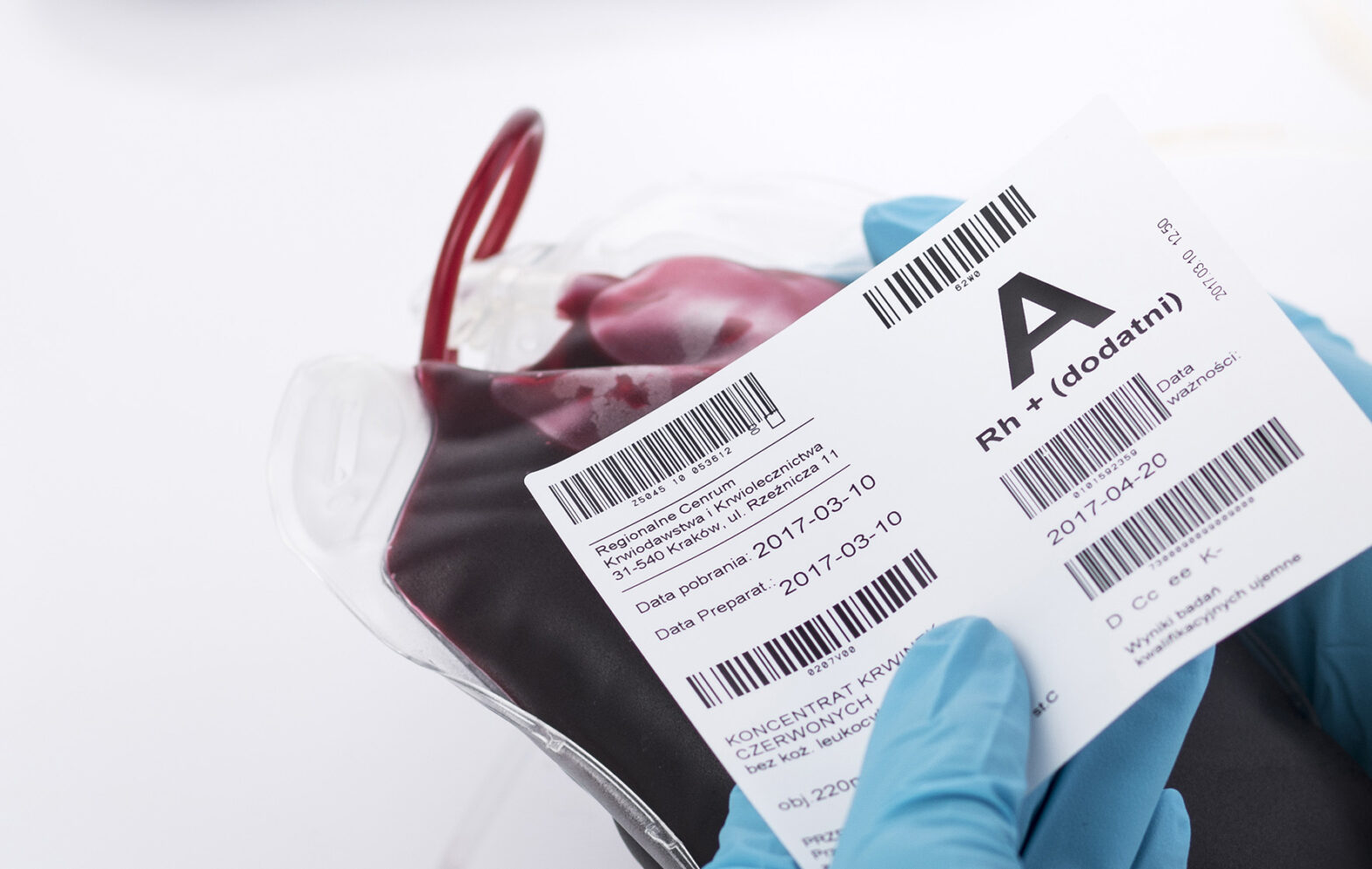
Printers with code verifier – minimize errors, maximize efficiency
Companies that invest in advanced technologies have the opportunity to stand out in the market. One example of such advances are label printers with a code verifier, which improve the […]